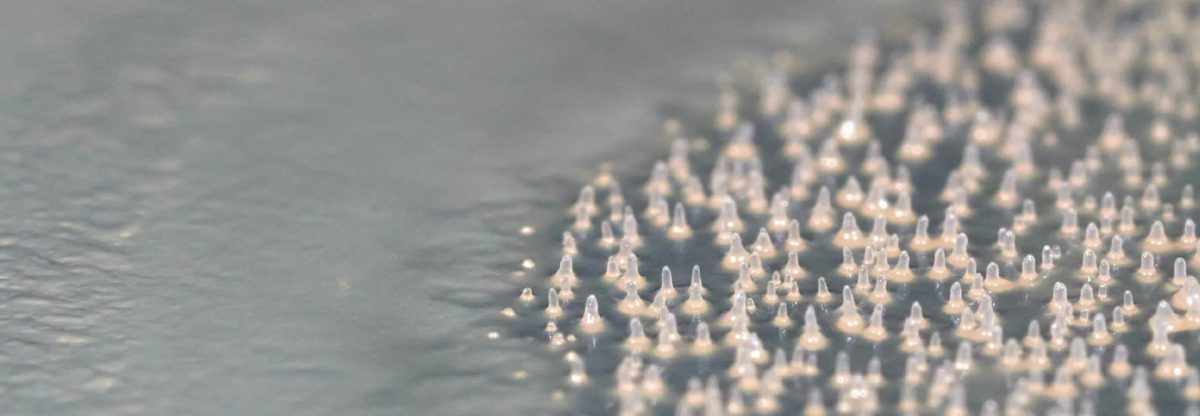
The poorly understood Fonticula alba, a relative of fungi and animals, hunts bacteria with a mechanism that resembles cancer and fungal growth.
Scientists have discovered that a slime mold can invade a colony of bacterial cells similar to how cancer cells invade healthy tissue. As reported in a new study, published Monday (March 28) in Current Biology, the invasive behavior of the slime mold, Fonticula alba, may hold clues as to how multicellular structures arose in fungi and animals.
F. alba, which has only ever been found in the environment one time (on some dog feces from Arkansas, back in the 1960s), drew little interest until a few years ago, when scientists from the University of Geneva became interested in its unique position on the evolutionary tree. While most previously characterized slime molds, such as Dictyostelium spp., are relatives of animals and plants, F. alba is instead more closely related to fungi than other slime molds.
“Fungi and animals are very closely related on the evolutionary tree,” Chris Toret, a microbiologist at the University of Geneva, tells The Scientist. “Why did the fungi end up becoming a fungi and the animal become an animal? There’s clearly some differences in how they stick together. And that’s why we wanted to look at [F. alba] as a model organism.”
Since little was known about F. alba, the first thing to do was to get acquainted with it. “I spent some time just trying to figure out, ‘can we grow this in lab?’” Toret explains. “Even just doing that, we started to see some interesting multicellular properties.”
Feeding time for slime
Previous studies had characterized some of F. alba’s life cycle. Like most slime molds, it spends most of its life in a single-cell form, as an amoeba feeding on bacteria. At some point in its life cycle, however, it becomes multicellular, joining with others into volcanolike fruiting bodies, which release spores and help the slime mold propagate. Previous researchers have described how, in this aggregated state, other slime molds can make decisions, and create patterns strikingly similar to subway systems.
But what went completely undescribed in the literature was F. alba’s other multicellular behavior: invasion. Toret and his colleagues found that F. alba only entered its aggregative state when cocultured with bacteria—in this case, the common fecal bacteria Klebsiella pneumonia—that’s in a particular phase of the bacterial life cycle. Bacteria go through phases of growth and death, growing quickly and then dying off once their food sources are depleted. It was exposure to bacteria in the scarcity-driven sunset phase that prompted the slime mold to aggregate, although the scientists don’t yet know why. The bacteria “needs to be mature like a good French cheese,” suggests Marko Kaksonen, a microbiologist at the University of Geneva and a coauthor of the paper.
The researchers found that in its invasive social state, F. alba sweeps through the depleting bacterial lawn, feasting and searching for new food sources as it goes. The researchers compared this invasive mechanism to how cancer cells collectively burrow into surrounding tissues and fungi’s use of long, branching filaments to creep along, searching for new sources of food, though the mechanisms by which F. alba invade bacterial colonies remain unknown. The slime mold cells form multicellular tendrils that extend through bacteria-filled agar, in which “follower cells” are guided by a single “leader” cell. In this state, the cells in the tendril communicate—when a leader cell enters a bacteria-free environment, it signals the cells following it to turn around. Cancer cells also use a coordinated, leader-follower configuration to migrate into adjacent tissues from the primary tumor.
The researchers used lasers to disrupt the movement of the leader cells, finding that zapping them disrupted the tendril’s movement and resulted in disordered motion. Eventually, other leader cells took up the mantle and the invasion proceeded. This didn’t happen when follower cells were zapped, however, indicating that F. alba cells are assigned distinct roles during this aggregative phase of their life.
“It’s a very important and interesting study,” Stuart Newman, a cell biologist at New York Medical College who was not involved in the study, tells The Scientist, “It’s a new phenomenon for aggregative microorganisms.”
Evolutionary links
Aggregation in slime molds has long fascinated scientists who study the origins of multicellularity—that is, how our single-celled ancestors came together to form tissues, eventually enabling the evolution of animals, mulicellular fungi, and plants.
As the researchers observed the slime mold’s tendrils, they noticed similarities to how fungi grow and explore, suggesting that the two share an evolutionary mechanism. Some scientists had hypothesized that hyphae, the filamentous structures that are the main mode of fungal exploration, evolved from neural outgrowths. But the invasive behavior of F. alba instead suggests that an “invasive hyphal network could have been built out of aggregative mechanisms,” says Toret.
“We think animals evolved being aggregative,” says Toret, “now we think arborized searching evolved aggregatively as well.”
Newman says that invasive social aggregation seems “similar to what’s going on in cancer” and that the study is evidence that “social cells do similar things . . . for different reasons.” He speculates that while different organisms may use different molecules to aggregate, collective invasion may be a generic property of social cells preserved across evolutionary lineages.
Moreover, Newman adds, the study shows that invasive behavior is “not dependent” on the same molecules across lineages, “which . . . goes against some of the strongly held ideas about evolution.
“Fonticula is an exceptionally cool and charismatic microorganism,” Matthew Brown, a microbiologist at Mississippi State University who didn’t work on the study, writes in an email to The Scientist. Though he describes the study as an “excellent paper,” and “an incredible observation,” he says he was “surprised” to see the comparison between collective invasion in cancer and F. alba, saying that it’s “a bit of a reach.” He says that the authors’ hypothesis of an evolutionary link between the collective invasion behavior in F. alba and fungi is “also a bit of a reach, just knowing that fungi are not amoeboid, fungi are all walled cells.”
However, he says, “It’s definitely a hypothesis that is worth testing.”
Author: Natalia Mesa
Reference: https://www.the-scientist.com/news-opinion/cancer-like-slime-mold-growth-hints-at-multicellularity-s-origins-69865